Laboratory Information System
Exploit the full potential of cloud-based laboratory information systems with Invitro LIS.
A complete, future-ready laboratory information system
Dedalus InVitro LIS stands as a cutting-edge, cloud-based Laboratory Information System offering comprehensive lab workflow management. Designed to cater to both single laboratories and multisite/multicampus organizations, it facilitates full integration with order entry systems, Laboratory Automation Systems (LAS), and result distribution platforms.
InVitro LIS main covered disciplines:
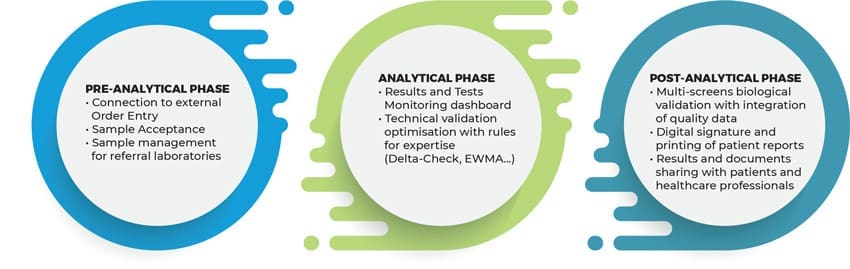
Dedalus InVitro Laboratory Information System capabilities support the full diagnostic process
Key Benefits
- Ultimate simplicity: no installation needed, simply connect and log in to access all your laboratory data and functionalities.
- Scalability by design: Continuous evolution through the consolidation of new disciplines thanks to the native use of standardized data.
- Excellent user experience: flexible and optimized workflows. Dashboards can be customized by user or role.
- Guaranteed performance and piece of mind, thanks to the unified LIS (Laboratory Information System) and LAS (Laboratory Automation System) customer service, providing end-to-end client support
- Simplified and reduced cost of integration with the healthcare ecosystem, via Dedalus DC4H ESB
- Reduced TCO, thanks to:
- Cloud technology, enabling “zero footprint” on clients
- Opensource database
Key features
- InVitro LIS provides flexibility and responsiveness to the laboratory organizational model supportingall the diagnostic process.
- InVitro LIS supports completely the ISO 15189 certification process providing:
- Equipment management
- Products Inventory
- Document management
- Personnel management
- Method validation
- Audit management
Product Information
FAQs
A Laboratory Information System (LIS) is a digital platform that manages data, workflows, and communications within a laboratory setting. It supports test ordering, sample tracking, result validation, and reporting, improving accuracy and efficiency. Learn more about our LIS solutions.
Dedalus LIS streamlines end-to-end laboratory workflows — from test requisitions to results delivery — enabling better data management, process automation, and regulatory compliance across clinical and diagnostic laboratories.
Implementing a LIS improves laboratory efficiency, minimises human error, enhances turnaround time, and provides clear audit trails — supporting better clinical decisions and laboratory performance.
Yes, Dedalus LIS is designed with interoperability in mind. It integrates seamlessly with hospital information systems (HIS), electronic health records (EHR), and other clinical applications to enable consistent, standardised data exchange across the care continuum.
Dedalus Labs brings decades of experience in clinical diagnostics and laboratory IT solutions. Their expertise underpins the development of high-performing, standards-based LIS platforms tailored to evolving healthcare needs.
It is possible to request a product demonstration or receive support by filling out the “Contact Us” form on this page or by contacting your Dedalus sales representative.
CONTACT US
Request a brochure, book a product demo or simply ask a question: our specialists are at your disposal to answer to all your needs or doubts.
"'*' indicates required fieldsLorem ipsum dolor sit amet, consectetur adipiscing elit. Sed in tellus vel lacus mollis dignissim congue vitae eros. Vestibulum neque nunc, eleifend vehicula accumsan et, faucibus a libero.
"*" indicates required fields
